PR Newswire
BEIJING, Oct. 15, 2024
- After 24 weeks of treatment in patients with Type 2 diabetes, the bi-weekly GLP-1 receptor agonist GZR18 injection showed superior efficacy in lowering HbA1c and body weight compared to semaglutide (Ozempic®).
- After 16 weeks of treatment in patients with Type 2 diabetes, the once-weekly insulin analog GZR4 injection demonstrated superior HbA1c reduction in patients with uncontrolled diabetes on basal insulins, compared to insulin degludec (Tresiba®).
- After 16 weeks of treatment in patients with Type 2 diabetes, the premixed dual insulin analog GZR101 injection showed superior efficacy in reducing HbA1c and postprandial glucose compared to insulin degludec/insulin aspart (Ryzodeg®).
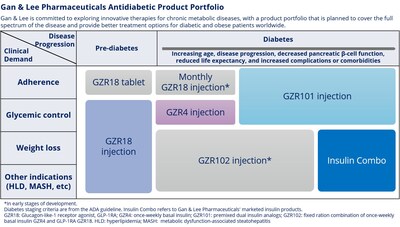
BEIJING, Oct. 15, 2024 /PRNewswire/ -- Recently, Gan & Lee Pharmaceuticals (Gan & Lee, Shanghai Stock Exchange: 603087) announced that three of its independently developed drugs — the bi-weekly GLP-1 receptor agonist GZR18 injection, the once-weekly basal insulin analog GZR4 injection, and the premixed dual insulin analog GZR101 injection — successfully achieved positive results in three Phase 2 clinical trials conducted in adult patients with Type 2 diabetes (T2D) in China. In these studies, Gan & Lee's three innovative drugs demonstrated superior or comparable efficacy in lowering glycated hemoglobin (HbA1c) compared to the respective positive comparator drugs separately.
Statement:
1. GZR18, GZR4, and GZR101 are investigational drugs that have not yet been approved in China.
2. Gan & Lee Pharmaceuticals does not recommend the use of any unapproved drugs/indications.
GZR18 Injection: a Phase 2 Study evaluate the Efficacy and Safety of GZR18 Injective vs. Semaglutide (Ozempic®) in Chinese Patients with Type 2 Diabetes
The Phase 2b clinical trial (CTR20232069) is a multicenter, randomized, open-label study comparing the efficacy and safety of GZR18 injection versus semaglutide (Ozempic®) in Chinese adult T2D patients with poor glycemic control on lifestyle interventions and/or unregulated use of anti-diabetic drugs, and on oral anti-diabetic treatment for at least three months. A total of 264 subjects were recruited across 25 clinical centers in China. Patients were randomized to receive bi-weekly GZR18 injections (12 mg, 18 mg, or 24 mg) or once-weekly 24 mg GZR18 injections, or 1 mg semaglutide for 24 weeks, including the dose-escalation period. The primary efficacy endpoint was the change in HbA1c from baseline after 24 weeks of continuous treatment. The subjects had a mean diabetes history of 11-18 years, and baseline HbA1c ranged from 8.28% to 8.56%.
After 24 weeks of treatment, the mean HbA1c reduction from baseline in the GZR18 groups was as follows: 1.87% (12 mg, bi-weekly), 2.28% (18 mg, bi-weekly), 1.94% (24 mg, bi-weekly), and 2.32% (24 mg, once-weekly), all higher than the semaglutide group (1.60% reduction)*. Additionally, in treatment-naïve patients with poor glycemic control on lifestyle interventions, the HbA1c reduction in the bi-weekly GZR18 injection group reached 2.98%, compared to 2.04% in the semaglutide group, with a significant HbA1c reduction (p < 0.05)*. After 24 weeks of treatment, patients in the bi-weekly GZR18 group experienced a maximum weight loss of 5.42 kg, compared to 3.25 kg in the semaglutide group*. GZR18 also greatly improved fasting glucose, blood pressure, and lipid levels, etc, providing comprehensive benefits for diabetic patients. GZR18 was generally well-tolerated, with safety and tolerability consistent with known GLP-1 receptor agonists. The most common adverse events were gastrointestinal-related, and no severe hypoglycemic events were observed.
GZR4 Injection: a Phase 2 Study Comparing the Efficacy and Safety of GZR4 injection vs. insulin degludec (Tresiba®) in Chinese Patients with Type 2 Diabetes
The Phase 2 trial (CTR20232431) was a multicenter, randomized, open-label, parallel-group study evaluating the efficacy, tolerability and safety of once-weekly GZR4 injections versus once-daily insulin degludec (Tresiba®) in 83 T2D patients with poor glycemic control on oral antidiabetic drugs (Part A) and 96 T2D patients with poor glycemic control on a combination of oral antidiabetic drugs and basal insulins (Part B).
In part A, the mean HbA1c reduction of once-weekly GZR4 injection was 1.50%, comparable to insulin degludec's reduction of 1.48%. In part B, GZR4 injection demonstrated a superior HbA1c reduction of 1.26%, compared to -0.87% for insulin degludec (treatment difference: -0.38%, p < 0.01)*. In addition, improvements in time-in-range (TIR) were comparable between GZR4 and insulin degludec. In this study, GZR4 injection showed good safety and tolerability, with no severe hypoglycemic events observed.
GZR101 Injection: a Phase 2 Study Comparing the Efficacy and Safety of GZR101 injection vs. insulin degludec/insulin aspart (Ryzodeg®) in Chinese Patients with Type 2 Diabetes
The Phase 2 clinical trial (CTR20232431) is a multicenter, randomized, open-label, parallel-controlled, treat-to-target Phase 2 study. Part A of the study aimed to compare the efficacy, safety, and tolerability of GZR101 injection administered once-daily versus insulin degludec/insulin aspart (Ryzodeg®) administered once-daily over 16 weeks in 62 patients with uncontrolled T2D on oral anti-diabetic drugs. In Part B, the study compared the efficacy, safety, and tolerability of GZR101 injection combined with insulin aspart versus insulin degludec/insulin aspart (Ryzodeg®) administered twice-daily over 16 weeks in 91 patients with uncontrolled T2D on basal/premixed insulin.
In Part A, the HbA1c levels in the once-daily GZR101 injection group decreased by 1.56%, superior to the -1.31% reduction in the once-daily insulin degludec/insulin aspart group (treatment difference: -0.24%). In Part B, GZR101 injection combined with insulin aspart achieved HbA1c reductions of -1.64% (once-daily insulin aspart) and -1.68% (twice-daily insulin aspart), both higher than the -1.59% reduction in the twice-daily insulin degludec/insulin aspart group (treatment differences of -0.06% and -0.09%, respectively)*. Moreover, GZR101 injection demonstrated comparable efficacy to insulin degludec/insulin aspart in controlling fasting and postprandial blood glucose, as well as improving time-in-range (TIR). In this trial, GZR101 injection showed good safety and tolerability. The estimated incidence of hypoglycemic events in both groups did not show statistically significant differences during the study.
*The trial results are presented as least squares means (LS mean).
Note: severe hypoglycemia is defined as Grade 3 hypoglycemia (serious events without specific plasma glucose boundaries, with conscious and/or somatic changes, hypoglycemia requiring help from others).
Dr. Zhong-ru Gan, Chairman of Gan & Lee Pharmaceuticals, stated:
"The positive results achieved by GZR18, GZR4, and GZR101 in Phase 2 clinical trials mark an important milestone in improving the current landscape of diabetes treatment. These results demonstrate that our three products provide better glycemic control compared to similar antidiabetic drugs.
Over the years, we have successfully launched a full range of insulin products, from human insulin to insulin analogs, covering both basal and mealtime insulin, in the hope of comprehensively meeting the treatment needs of different diabetes patients. With recent breakthroughs in our research and development, we have also expanded our product portfolio with oral antidiabetic drugs, filling a gap in this critical area.
Focusing on the frontiers of the endocrine field, we are actively promoting the development of new drugs, aiming to achieve comprehensive management of chronic metabolic diseases, including diabetes and obesity. Gan & Lee's product portfolio currently covers next-generation insulin based on the extension to emerging metabolic disease targets, such as GLP-1 receptor agonists, supporting patients throughout their treatment journey with a view to driving changes in the management of diabetes and metabolic diseases and improving the quality of life of patients."
Forward-Looking Statements:
These forward-looking statements are based on expectations and assumptions as of the date of this release. Due to various factors, actual results may differ significantly. We do not undertake any obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
Reference
1. WANCAI XING, WEI CHEN, YINING ZHANG, JING GAO, ANSHUN HE, JUN ZHANG, YING DENG, FANGKAI XUE, YONGCHUN WANG, HAO FU, RUNYAO ZHANG, JINHUI HUANG, ZHONGRU GAN; 823-P: Molecular and Pharmacological Properties of GZR4, a Once-Weekly Insulin Analog. Diabetes 14 June 2024; 73 (Supplement_1): 823–P. https://doi.org/10.2337/db24-823-P
About Gan & Lee
Gan & Lee Pharmaceuticals developed the first Chinese domestic insulin analog. Currently, Gan & Lee has six core insulin products, including five insulin analog varieties: long-acting glargine injection (Basalin®), fast-acting lispro injection (Prandilin™), fast-acting aspart injection (Rapilin®), mixed protamine zinc lispro injection (25R) (Prandilin™25), aspart 30 injection (Rapilin®30), and one human insulin injection - mixed protamine human insulin injection (30R) (Similin®30). The company has two approved medical devices in China, namely reusable insulin injection pen (GanleePen), and disposable pen needle (GanleeFine®).
In China's 2024 National Insulin-Specific Centralized Procurement, Gan & Lee Pharmaceuticals ranked first among all selected companies in terms of procurement demand for insulin analogs. The company is also making strides in international markets, with the disposable pen needle (GanleeFine®) approved by the US Food and Drug Administration (FDA) in 2020 and received GMP inspection approval from the European Medicines Agency (EMA) in 2024. These achievements significantly boost Gan & Lee's competitiveness in both international and domestic markets.
In the future, Gan & Lee will strive for comprehensive coverage in diabetes treatment. Moving forward with its mission to become a world-class pharmaceutical company, Gan & Lee will also actively develop new chemical entities and biological drugs, focusing on treatments for metabolic diseases, cardiovascular diseases, and other therapeutic areas.
Further Information:
[email protected] (Media)
[email protected] (Business Development)
[email protected] (Medical Information)
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/gan--lee-pharmaceuticals-three-innovative-drugs-gzr18-injection-gzr4-injection-and-gzr101-injection-achieve-primary-endpoints-in-phase-2-clinical-studies-302276311.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/gan--lee-pharmaceuticals-three-innovative-drugs-gzr18-injection-gzr4-injection-and-gzr101-injection-achieve-primary-endpoints-in-phase-2-clinical-studies-302276311.html
SOURCE Gan & Lee Pharmaceuticals